Researchers in the United States have analyzed the neutralizing activity of Moderna’s mRNA-1273 vaccine against several variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19).
“The emergence of SARS-CoV-2 variants has led to growing concerns over increased transmissibility and the ability of some variants to partially escape immunity,” writes the team from Moderna Inc. in Cambridge, Massachusetts.
Now, the researchers have compared the neutralizing activity of sera from individuals immunized with the mRNA-1273 vaccine against emergent variants and the wild-type SARS-CoV-2 virus (designated D614G).
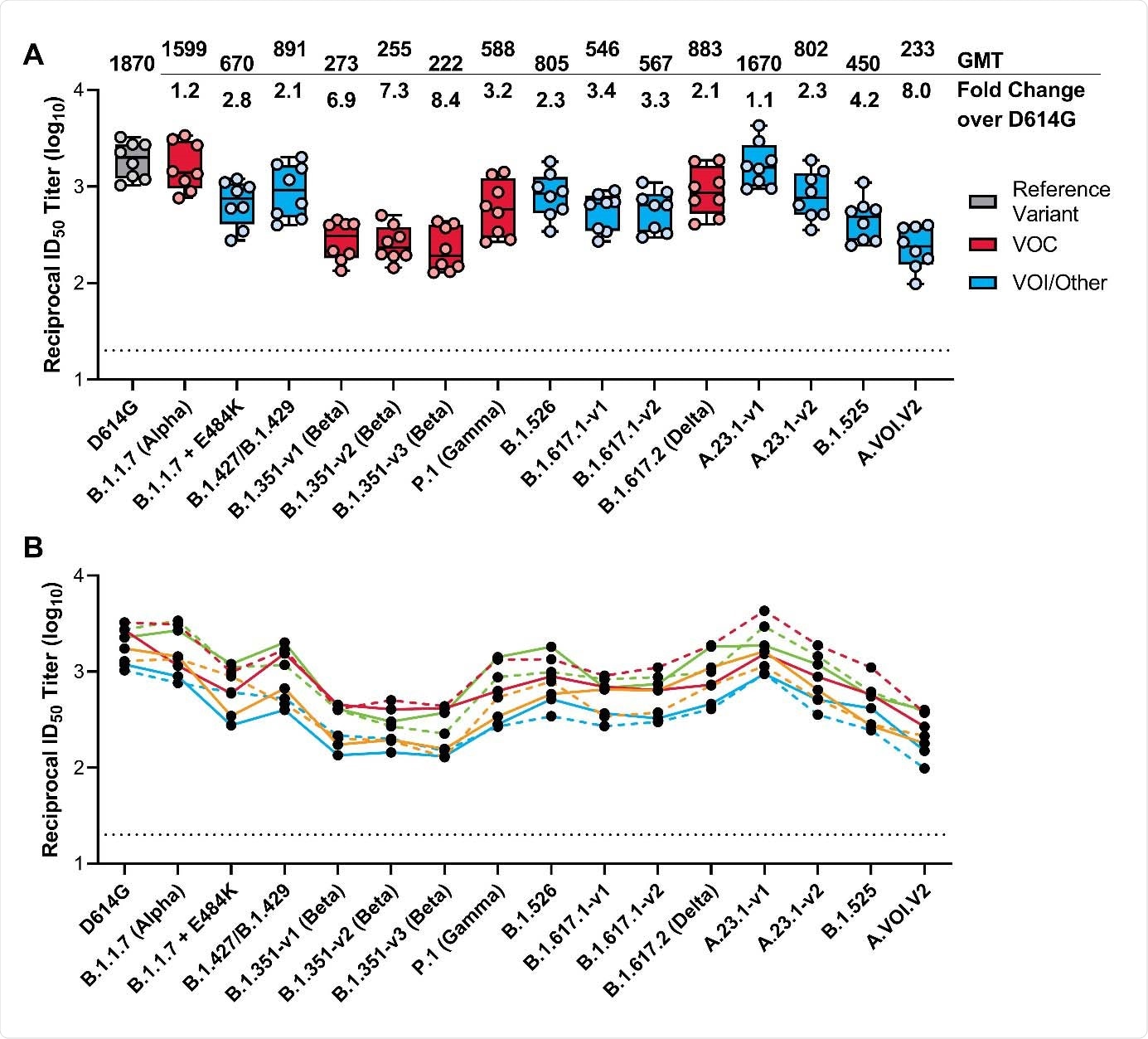
The team reports that vaccine-elicited neutralizing antibody titers against the B.1.1.7 (Alpha) variant of concern were comparable to those elicited against D614G.
By contrast, reduced neutralization titers were observed for all other variants of concern, including the B.1.351 (Beta), B.1.617.2 (Delta), and P.1 (Gamma) lineages, with reductions ranging from 2.1- to 8.4-fold.
However, all variants remained susceptible to mRNA-1273-elicited serum neutralization.
Darin Edwards and colleagues say the findings highlight the need to continually assess the protection conferred by mRNA-1273 against emergent variants to inform any vaccine modifications that are needed in the future.
“This may help to mitigate the ongoing spread of SARS-CoV-2 and the emergence of new variants,” they write.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
.jpg)
Emergent variants pose a threat to vaccination efforts
Since the COVID-19 outbreak first began in late December 2019, several SARS-CoV-2 variants of interest (VOIs) and variants of concern (VOCs) have emerged. These lineages contain mutations that confer increased transmissibility and partial escape from infection or vaccine-induced immunity.
The variants include those that emerged in the United Kingdom (B.1.1.7; Alpha), South Africa (B.1.351; Beta), Brazil (P.1; Gamma), India (B.1.617.1, Kappa; B.1617.2, Delta), Nigeria (B.1.525; Eta), the United States (B.1.526; Iota), Uganda (A.23.1), and Angola (A.VOI.V2).
Importantly, the B.1.617.2 lineage has been associated with an increased transmission rate, reduced efficacy of monoclonal antibody treatment, and reduced susceptibility to neutralizing antibodies.
Edwards and colleagues previously reported that Moderna’s mRNA-1273 vaccine, which is based on the original SARS-CoV-2 strain isolated in Wuhan in China, elicited high neutralizing antibody titers among phase 1 clinical trial participants and was highly effective at preventing symptomatic and severe COVID-19.
However, another study Edwards was involved in found that some VOCs or VOIs, including B.1.351 and P.1, reduced neutralizing antibody titers in a pseudovirus-based model.
What did the current study involve?
“Here we provide an update on the neutralization activity of vaccine sera against several newly-emerged variants, including the Delta variant B.1.617.2,” says the team.
The researchers used sera samples collected from eight phase 1 clinical trial participants who had received 100µg of the mRNA-1273 vaccine on a prime-boost regimen.
Neutralizing activity against several SARS-CoV-2 variants, including VOCs and VOIs, was compared with neutralizing activity against the wild-type virus (D614G).
What were the results?
The B.1.1.7 variant only had a minimal effect on neutralizing antibodies titers, which were reduced by just 1.2-fold compared with titers against D614G.
The neutralization titers for all other variants, including B.1.351 (Beta) B.1.617.2 (Delta), and P.1 (Delta) decreased by a factor of 2.1 to 8.4, compared with titers against D614G.
However, all variants remained susceptible to mRNA-1273–elicited serum neutralization, says the team.
Across three versions of the B.1.351 variant (B.1.351-v1, B.1.351-v2, and B.1.351-v3), reductions in neutralization titers ranged from 6.9-fold to 8.4-fold.
Of all the variants tested, the most significant effect on neutralization titers was observed for A.VOI.V2 and B.1.351-v3, which decreased by 8.0-fold and 8.4-fold, respectively.
The effect on neutralizing activity was more modest for P.1 and B.1.617.2, with titers falling by 3.2- and 2.1-fold, respectively.
What did the authors conclude?
“These data emphasize the need to continually assess the ability of mRNA-1273 to confer protection against prevalent and emergent VOIs and VOCs,” says Edwards and the team.
“Such data are crucial to inform necessary modifications to COVID-19 mRNA vaccines going forward, which may help to mitigate the ongoing spread of SARS-CoV-2 and the emergence of new variants,” they conclude.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Edwards D, et al. Serum Neutralizing Activity of mRNA-1273 against SARS-CoV-2 Variants. bioRxiv, 2021. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.06.28.449914, https://www.biorxiv.org/content/10.1101/2021.06.28.449914v1
Posted in: Drug Trial News | Medical Research News | Disease/Infection News
Tags: Antibodies, Antibody, Assay, Clinical Trial, Coronavirus, Coronavirus Disease COVID-19, Efficacy, immunity, Monoclonal Antibody, Pseudovirus, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Stomatitis, Syndrome, Vaccine, Virus

Written by
Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Source: Read Full Article
