The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genome generates 16 distinct non-structural proteins (nsp) that constitute the enzymes and accessory proteins responsible for virus replication once inside a host cell. These protein complexes produce and cap RNA strands that will go on to be translated by the host machinery. Capping the RNA in a manner that is recognized as similar to endogenous mRNA ensures compatibility, lessens RNA degradation rates and lowers the probability of triggering an immune response in the host cell.
In a paper recently uploaded to the preprint server bioRxiv* by Diffley et al. (April 8th, 2021), inhibitors of one essential SARS-CoV-2 non-structural protein are explored, with four compounds, in particular, identified as potential antiviral leads that exhibit synergistic effects with antiviral drug remdesivir.
.jpg)
The function of nsp14
Following synthesis of the viral RNA strand, nsp13 is involved in preparing the strand for capping by removing the terminal phosphate group, after which nsp12 transfers the GpppA cap structure to the 5’ end of the RNA. Nsp14, nsp16, and nsp10 then finalize the cap structure by adding several methyl groups, forming Cap1.
The group demonstrated that methylation performed by the nsp16/nsp10 complex is dependent upon the prior methylation of the strand by nsp14, and both of these enzymes require the presence of S-adenosyl-L-methionine (SAM), a methyl donor, producing S-adenosyl-L-homocysteine (SAH) upon donation. Nsp14 was shown to be a guanine-N7 methyltransferase that can only catalyze the methylation of the starting GpppA structure.
Nsp14 was purified and the group developed an assay to quantifiably determine the conversion of SAM to SAH in the presence of the enzyme and GppA-capped RNA, thereby determining the activity of the methyltransferase enzyme. Nsp14 was also screened against a library of over 5,000 chemical compounds in search of antiviral drug leads, applied at a concentration of 3.125 μM, and each ranked based on efficacy. The resulting drug leads were further refined by removing probable screening errors, compounds that would be difficult to source, or those with known toxicities at the indicated required concentration.
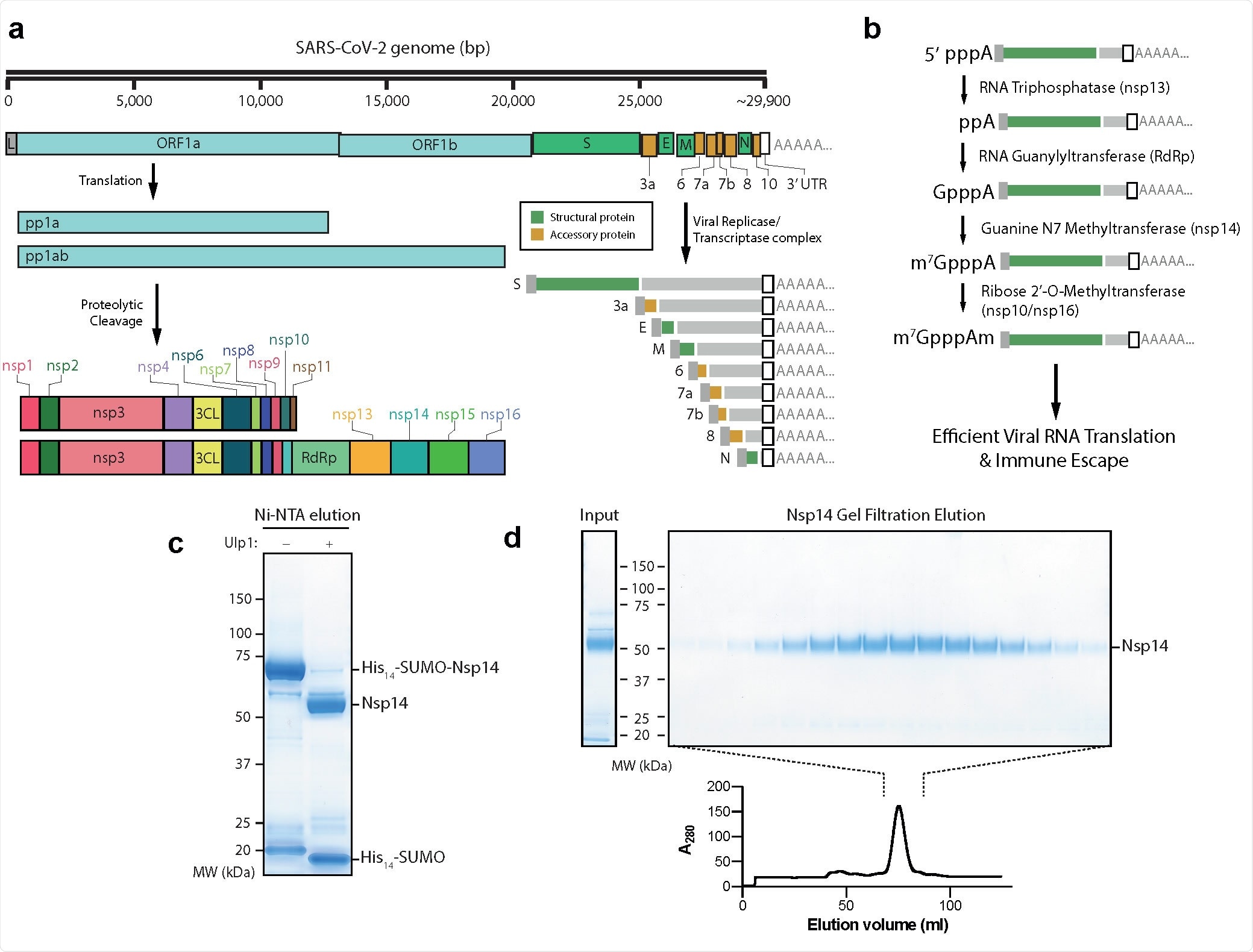
Nsp14 inhibitors
A chemical compound termed PF-03882845 was found to be the most potent inhibitor, exhibiting an inhibitory concentration 50% of 1.1 μM. Trifluperidol, Inauhzin, and Lomeguatrib had IC50 values of 12.9 μM, 23 μM, and 59.8 μM, respectively. The activity of these drugs towards the nsp16/nsp10 complex was also tested, and none were found to inhibit the complex, demonstrating the specificity of the drugs towards nsp14.
Mammalian cells were then infected with SARS-CoV-2, and the inhibitors applied, followed by fluorescent staining of anti-nucleoprotein antibodies, allowing the group to track both viral load and cytotoxicity towards the cells. PF-03882845, Inauhzin, and Trifluperidol each had IC50 values of between 11-13 μM, in order of lessening potency, while Lomeguatrib required a concentration of almost 60 μM for similar inhibition levels. None of the drugs proved to be cytotoxic in the cell line tested.
Antiviral drug remdesivir generally requires concentrations of around 1 μM to lower viral load in the cells tested by the group. To test synergy, the drug was applied at a concentration of only 0.5 μM in combination with one of the four lead drugs. In this case, Inauhzin exhibited similar IC50 values when applied alone, while the three other drugs exhibited significant synergy, having notably lower IC50 values. The IC50 of PF-03882845 and Trifluperidol lowered in particular, dropping to 4.79 μM and 5.05 μM, respectively.
PF-03882845 and Inauhzin have previously been in clinical trials as a mineralocorticoid receptor agonist and SIRT inhibitor, respectively, though neither has yet been approved for human use. Trifluperidol is a licensed therapeutic used to treat various psychoses but has been implicated with various detrimental side effects under long-term use, and so may not make an ideal COVID-19 prophylactic. In any case, these drugs may contribute to the development of more effective SARS-CoV-2 drugs.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Identification of SARS-CoV-2 Antiviral Compounds by Screening for Small Molecule Inhibitors of the nsp14 RNA Cap Methyltransferase, Souradeep Basu, Tiffany Mak, Rachel Ulferts, Mary Wu, Tom Deegan, Ryo Fujisawa, Kang Wei Tan, Chew Theng Lim, Clovis Basier, Berta Canal, Joseph F. Curran, Lucy Drury, Allison W. McClure, Emma L. Roberts, Florian Weissmann, Theresa U. Zeisner, Rupert Beale, Victoria H. Cowling, Michael Howell, Karim Labib, John F.X. Diffley, bioRxiv, 2021.04.07.438810; doi: https://doi.org/10.1101/2021.04.07.438810, https://www.biorxiv.org/content/10.1101/2021.04.07.438810v1
Posted in: Medical Research News | Disease/Infection News
Tags: Antibodies, Antiviral Drug, Assay, Bases, Cell, Cell Line, Compound, Coronavirus, Coronavirus Disease COVID-19, Cytotoxicity, Drugs, Efficacy, Enzyme, Genome, Guanine, Homocysteine, Immune Response, Mammalian Cells, Methionine, Molecule, Nucleotide, Protein, Receptor, Remdesivir, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Structural Protein, Syndrome, Virus

Written by
Michael Greenwood
Michael graduated from Manchester Metropolitan University with a B.Sc. in Chemistry in 2014, where he majored in organic, inorganic, physical and analytical chemistry. He is currently completing a Ph.D. on the design and production of gold nanoparticles able to act as multimodal anticancer agents, being both drug delivery platforms and radiation dose enhancers.
Source: Read Full Article
