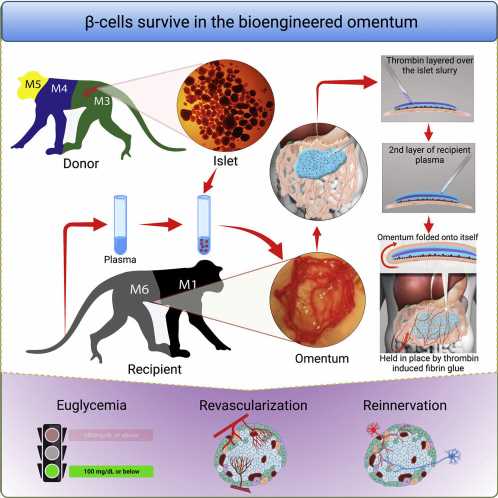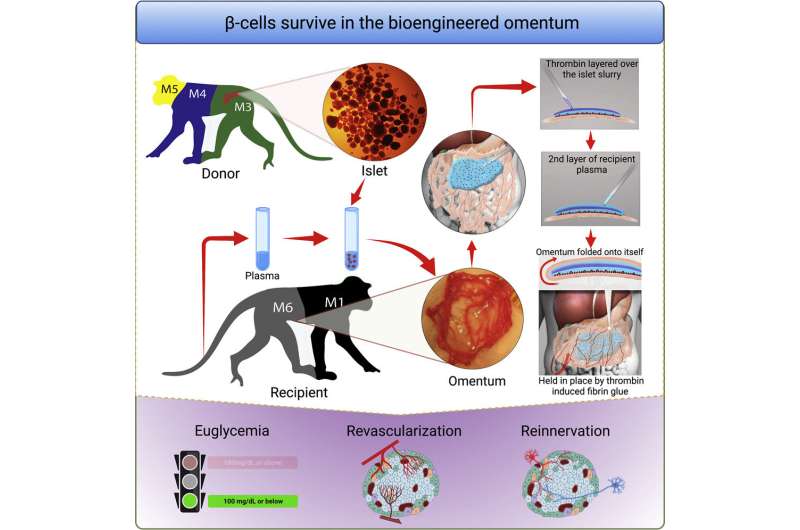
In people with type 1 diabetes, the body’s immune system attacks and destroys insulin-producing β cells that control blood glucose levels and are part of a group of cells in the pancreas called pancreatic islets. In research published in Cell Reports Medicine, a team led by investigators at Massachusetts General Hospital (MGH), a founding member of Mass General Brigham, recently developed an efficient way to transplant pancreatic islets and demonstrated that the method can effectively reverse type 1 diabetes in nonhuman primates.
Pancreatic islet transplantation is a promising treatment approach for type 1 diabetes; however, current methods, which involve transplanting islets to the liver, are inefficient and can result in the loss of as much as half of transplanted β cells due to immune attack. Also, the liver can only accommodate a limited volume of transplanted tissue.
Scientists have wondered if an alternative site might provide a more hospitable environment and lead to better results. One promising site is the omentum, the fatty tissue that starts in the stomach and drapes over the intestines.
To optimize the omentum as a transplant site in an individual, investigators used topical recombinant thrombin (which stops bleeding), an enzyme, and the recipient’s own plasma to engineer a bio-degradable matrix by which donor islets are immobilized onto the omentum. When this strategy was used along with an immunosuppressive therapy to protect islets from immune attack, the method normalized blood glucose levels and restored glucose-responsive insulin secretion in three nonhuman primates with type 1 diabetes for as long as the animals were tested.
“The achievement of complete glycemic control is attributed to the bioengineering approach that facilitates the process of revascularization and reinnervation for the transplanted islets,” says first author Hong Ping Deng, MD, MSc, a researcher of Transplant Surgery at MGH. “which is the first time that such a demonstration has been made in a nonhuman primate model.”
“This pre-clinical study can inform the development of new strategies for β cell replacement in diabetes and could change the current paradigm of clinical pancreatic islet transplantation,” says senior corresponding author Ji Lei, MD, MBA, MSc, a principal physician investigator of Transplant Surgery at MGH and an assistant professor of Surgery at Harvard Medical School. “A clinical trial is being planned to test this approach.”
Lei, who is also the director of the Human Islet/Cell Processing Special Service cGMP Facility at MGH, notes that in addition to transplanting islets from donors, researchers are also studying the potential broad application of transplanting stem cell–derived islets, which cured a patient with type 1 diabetes for the first time in human history in 2022 and could offer an endless supply of transplantable tissue. There are concerns about this approach, however, including the possibility of tumor development.
Unlike the liver, the omentum is easily accessible for monitoring purposes, and its non-vital site status can allow for the removal of transplanted tissue should complications occur, with either stem cell–derived islets or islets from donors. In addition, the engineered omental site can be home to many other types of genetically engineered cells, especially for liver-based or inherited metabolic or endocrine disorders.
Co-author James F. Markmann, MD, Ph.D., chief of the Division of Transplant Surgery and director of Clinical Operations at the Transplant Center at MGH, stresses that the non-human primate study is a highly translational pre-clinical animal model. “The application of this strategy, particularly in stem cell–based therapy, has the potential to revolutionize the paradigm for treating patients with type 1 diabetes,” he says.
More information:
Hongping Deng et al, Bioengineered omental transplant site promotes pancreatic islet allografts survival in non-human primates, Cell Reports Medicine (2023). DOI: 10.1016/j.xcrm.2023.100959
Journal information:
Cell Reports Medicine
Source: Read Full Article