
The European Union on Monday authorised the Moderna coronavirus jab, giving the bloc a second vaccine and a shot in the arm after a slow start to its bid to protect its 450 million residents.
The sluggish rollout of the first vaccine made by Pfizer-BioNTech has drawn widespread criticism, as many of the bloc’s 27 countries battle surging infection rates.
The European Commission formally backed US firm Moderna’s vaccine hours after the Amsterdam-based European Medicines Agency (EMA) recommended conditional marketing approval for people over 18.
“We are providing more COVID-19 vaccines for Europeans. With the Moderna vaccine, the second one now authorised in the EU, we will have a further 160 million doses. And more vaccines will come,” European Commission chief Ursula von der Leyen said.
EU health commissioner Stella Kyriakides said that approval of the Moderna and Pfizer-BioNTech vaccines “will ensure that 460 million doses will be rolled out with increasing speed in the EU”.
Europe is lagging far behind the United States, Britain and Israel in terms of numbers inoculated.
National capitals have been piling pressure on the EMA, which brought forward an initial meeting to decide on the Moderna vaccine to Monday from January 12, but then said it had to clarify “outstanding issues.”
“This vaccine provides us with another tool to overcome the current emergency,” EMA Executive Director Emer Cooke said in a statement after it met again on Wednesday to approve the vaccine.
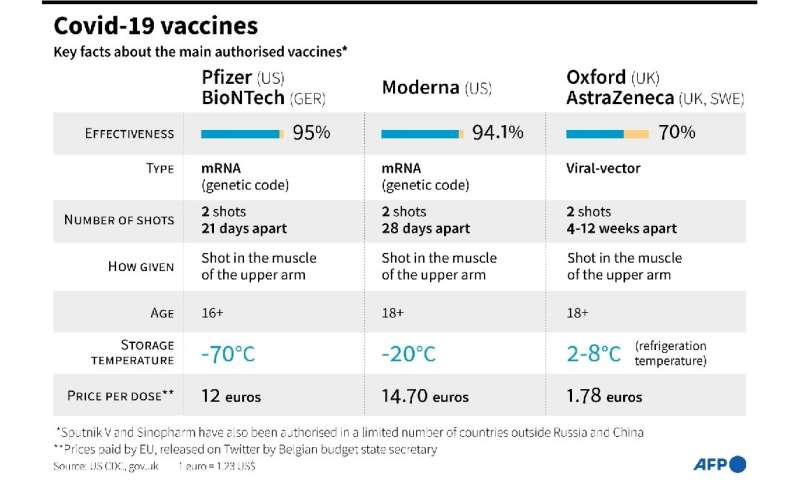
The Moderna vaccine is easier to store and transport than the Pfizer vaccine, which needs to be kept in freezers at a temperature of minus 70C, although it is more expensive.
The EMA’s one-year authorisation is for two injections of the Moderna vaccine into the arm, 28 days apart.
‘Gigantic challenge’
The EMA said its human medicines committee, with experts drawn from all 27 countries, had “thoroughly assessed the data on the quality, safety and efficacy of the vaccine” and recommended authorisation “by consensus”.
“This will assure EU citizens that the vaccine meets EU standards,” it added.
The EU insists it has taken the safest route in subjecting the candidate vaccines to full EMA testing protocols and coordinating closely with member states to decide how many doses to buy and distribute.
But anger is growing in countries around the bloc about the slow pace of the vaccinations since they started on December 27, after the Pfizer vaccine was approved on December 21.
The Netherlands on Wednesday became the final country in the bloc to start.
European Council chief Charles Michel said late on Tuesday that leaders would hold a virtual summit on the health crisis later this month.
Michel said delivering vaccines to the EU’s almost 450 million people was a “gigantic challenge”.
But he insisted the European Commission was “working night and day” to make more vaccines available.
Moderna’s jab was found to be 94.1 percent effective in clinical trials and the United States already uses it alongside the Pfizer-BioNTech vaccine.
Britain is using the Pfizer jab and another developed by UK pharmaceuticals giant AstraZeneca and Oxford University.
Source: Read Full Article
