On the road, putting a foot on the gas or brake pedal controls the car’s speed. This also happens in our body, in driving an immune response. Like human drivers, antibodies have a “foot”—a sort of molecular limb that “presses” the gas and brake pedals. Those pedals are receptors on the outer membranes of immune cells: When an antibody foot binds to one of these pedals, it can either speed up the immune response or slow it down. Among their other uses, antibodies are employed in a new generation of cancer treatments known as immunotherapy, which harness the immune system’s natural capabilities to fight malignant tumors. And as far as these cancer-fighting antibodies are concerned, speed is of the essence.
A new study, conducted by a team of researchers headed by Dr. Rony Dahan of the Weizmann Institute of Science, revealed that a small molecular change in a common immunotherapy antibody might enable it to bind better to certain “gas pedal” receptors, thus accelerating the immune response against cancer. The study, published Friday in Science Immunology, also shows that adding a second antibody—one that blocks inhibitory receptors—may improve the effectiveness of anticancer treatments.
In 2016, the United States Food and Drug Administration approved a breakthrough immunotherapy treatment that uses antibodies to block a protein called PD-L1. Cancer cells can exploit PD-L1 to suppress the immune response against them by “exhausting” the T cells that fight the cancer. The antibodies in current use were designed to operate in a direct manner: They neutralize PD-L1, thus preventing it from binding to T cells and exhausting them.
In a previous study, Dahan discovered that antibodies to PD-L1 could also act in an indirect manner, not only neutralizing the protein but also binding to receptors on immune cells and activating them against the cancer cells expressing PD-L1. That study, which suggested that the antibodies enhanced the treatment’s effectiveness, was carried out with mouse antibodies, rather than the human version used in cancer treatments.
In the new study, Dahan and his team in Weizmann’s Systems Immunology Department checked whether the findings are valid for the drugs meant for humans. To this end, the scientists used so-called humanized mice, in which genetic engineering was used to replace mouse antibody receptor genes with human ones. After inducing tumors in these mice, the scientists treated them with two clinically used antibodies: one that cannot bind immune cell receptors (the drug atezolizumab) and one that can (the drug avelumab).
Led by research student Noy Cohen Saban, the team followed the growth rate of tumors in the two groups of mice. Since the scientists knew that the binding antibodies also activated cancer-fighting immune cells, they were surprised to discover that there was no significant difference between the groups. Why did the human version of the drug not perform as it had in regular mice?
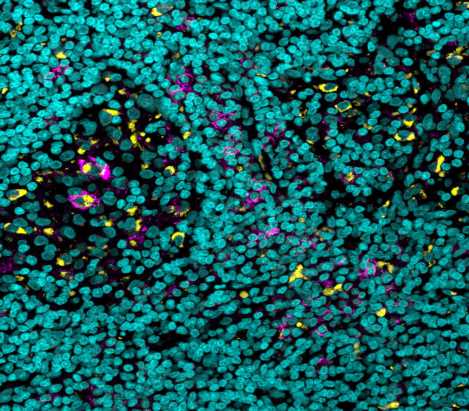
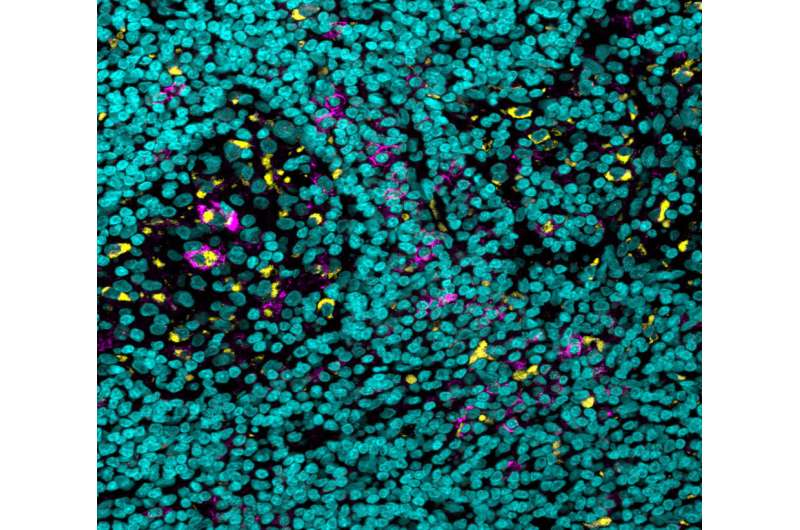
The researchers knew from past studies that, while the binding of antibodies to most receptors acts as a gas pedal that enhances the immune response, there is one receptor that acts as a brake pedal, and binding to this one inhibits the response. A closer look revealed that, compared with similar cells of other organs, there were many more of these brake pedal receptors on certain immune cells in the tumor’s microenvironment. That phenomenon was observed in human tumors as well. In tumor samples of skin and kidney cancers, which were obtained through the University of Michigan, the scientists identified an increased expression of the immune-suppressing receptors.
In other words, despite the drug’s boost to the immune system, the total effect was something like pressing the gas and brake pedals at the same time. Once the scientists realized what was happening, they tried the experiment again, this time giving the mice a combined treatment of avelumab and a second antibody that had been proven to inhibit the immune-suppressing receptors. With the foot lifted from the immune system’s brakes, the cancer treatment was much more effective.
The researchers thought they might be able to make the antibodies even more effective by getting them to press harder on the “gas pedals”—that is, they looked for a way to make the antibodies’ “feet” bind more tightly to the immune-enhancing receptors. They created a small change in a sugar molecule associated with the antibody’s foot—a change that can increase the binding affinity of an antibody elevenfold. And indeed, following treatment with the new, improved antibodies, the size of the tumors in humanized mice was smaller, and the treated mice’s average survival time was longer.
Finally, the team delved into the mechanism of action responsible for the success of their improved antibody. They found that this antibody gave the anticancer treatment a double advantage: It was able to both increase the numbers of T cells penetrating the tumor and to decrease the numbers of certain myeloid cells—immune cells that inhibit the anticancer response in the tumor’s microenvironment.
“The findings of this research could undergo a rapid transition from the laboratory to the clinic, to improve those drugs already available to cancer patients,” says Dahan. “Furthermore, the discovery of improved antibodies that act on immune cells other than T cells creates an opportunity to use them in treatments for certain still incurable cancers in which T cell-targeted therapies are ineffective.”
More information:
Noy Cohen Saban et al, Fc glycoengineering of a PD-L1 antibody harnesses Fcγ receptors for increased antitumor efficacy, Science Immunology (2023). DOI: 10.1126/sciimmunol.add8005
Journal information:
Science Immunology
Source: Read Full Article