
Offering population-wide genetic screening for risk of deadly but preventable diseases to young adults would save lives and be cost-effective in Australia, a Monash University-led study has found.
The study used health economic evaluation to model combined DNA screening for all adults in Australia aged 18–40 years, to identify risk of three different genetic conditions:
- Hereditary breast and ovarian cancer (caused by the BRCA1 and BRCA2 genes).
- A type of genetic high cholesterol called familial hypercholesterolemia.
- Lynch syndrome, a genetic condition that increases the risk of colorectal and other cancers.
All three conditions can be treated or prevented if detected early. Around 1 in 75 people are at high genetic risk of one of these conditions, but most are unaware and therefore not accessing available life-saving interventions.
Published in eClinicalMedicine, the modeling study used previous Australian and international evidence to extrapolate whether widespread preventive DNA screening for multiple diseases together would be cost-effective in the Australian public health care system, compared with the status quo of targeted clinical criteria-based testing.
The study found that offering population-level preventive testing to all 18- to 40-year-olds would be cost-effective in Australia, compared with the current practice of limited genetic testing.
Co-first author Associate Professor Paul Lacaze, from the Monash University School of Public Health and Preventive Medicine, said, “This modeling forms a critical pillar of evidence to support the development of a new DNA-based population screening program in Australia for cancer and heart disease risk.
“We can now say with confidence, based on our rigorous modeling analysis, that offering population-wide preventive DNA screening to young adults in Australia would not only save lives but also be cost-effective.
“Offering this type of DNA screening would finally maximize the preventive potential of genomics, and deliver many public health benefits to the Australian population.”
Over the simulated model of 8.3 million adults’ lifetime to age 80, assuming 50% uptake of DNA screening, an estimated 4,047 deaths could be averted. It would also prevent 2,612 cancers (1,140 breast, 950 ovarian, 451 colorectal and 71 endometrial) and 542 non-fatal coronary heart disease (CHD) events.
This is equal to preventing 63 cancers, 31 CHD cases and 97 deaths averted per 100,000 people offered DNA screening, versus current practice.
At $200 per test, a total government investment of $832 million would be required to screen 50% of the model population. This investment would also cover $539 per high-risk person identified, for clinical confirmation testing and genetic counseling.
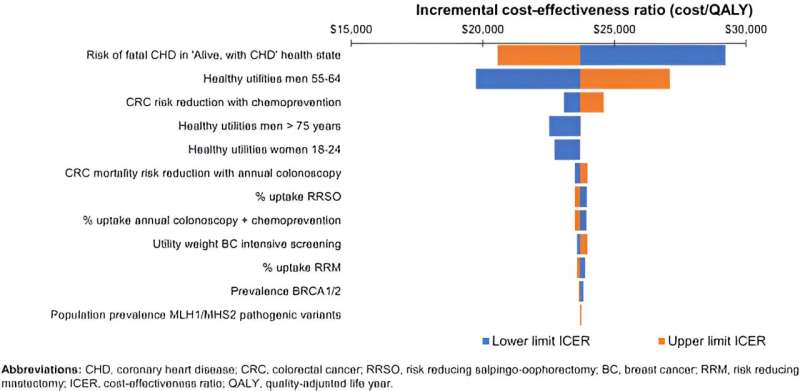
The study found the initial cost would be well worth it, given the overall benefit to society through early detection and prevention, with an incremental cost-effectiveness ratio (ICER) of AU$23,926 per quality-adjusted life-year (QALY) gained, as a result of the population genomic screening.
Senior author Professor Zanfina Ademi, who leads Monash University’s Health Economics and Policy Evaluation Research Group in the Faculty of Pharmacy and Pharmaceutical Sciences, said that combined population genomic screening for high-risk conditions in the young adult population may be cost-effective from the public health care perspective, compared to the current status-quo.
Professor Ademi said this was based on the willingness-to-pay threshold of AU$50,000—the amount the health care system is willing to spend per quality-adjusted life year gained.
“If you looked at it from a societal perspective, with potential health care costs and costs of lost work productivity considered, combined genomic screening would save lives and money compared to the status quo,” she said.
“This societal perspective is often considered in cost-effectiveness calculations conducted overseas.
“This further emphasizes the importance of allocating scarce resources to preventive health so that society gains overall in the long term from combined genomic screening, rather than testing or screening for single conditions individually, which is current practice.”
Co-first author Dr. Clara Marquina, a Faculty of Pharmacy and Pharmaceutical Sciences, Adjunct Research Fellow, said the state-of-art cost-effectiveness modeling techniques had provided evidence to inform policy and funding that could reduce the burden of disease.
“Using best practice economic evaluation methods is key to informing successful policy, implementation and scaling up of health care technologies,” she said.
The modeling in this paper used the same genes and health conditions as a 2022 world-first DNA Screen pilot study, which has almost completed offering genomic screening to 10,000 young adults aged 18–40 in Australia. Results and outcomes of the pilot will be available in 2024.
“Australia has a proven ability to deliver population-based screening programs through our national public health care system,” said Associate Professor Lacaze, who is also chief investigator of the DNA Screen pilot study. “These successful programs are guided by our established National Population-Based Screening Framework.
“Under these circumstances, the prospect of implementing a national DNA-based population screening program is feasible and exciting in Australia. Other matters, including psychosocial impacts, ethical and societal issues, and implementation challenges, also need consideration.
“We highlight the need for more public education and awareness of genomics; an appropriate informed-consent framework for population genomic screening to discuss potential benefits and harms, and the protection of high-risk individuals against genetic discrimination through new legislation in Australia.”
More information:
Paul Lacaze et al, Combined population genomic screening for three high-risk conditions in Australia: a modelling study, eClinicalMedicine (2023). DOI: 10.1016/j.eclinm.2023.102297
Journal information:
EClinicalMedicine
Source: Read Full Article
