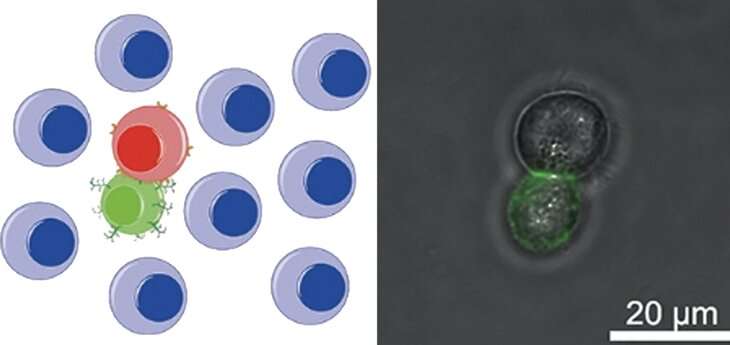
In cell-based immunotherapy, a treatment being explored for cancer, the body’s immune cells are modified to eliminate cancer cells more effectively. Immune cells can kill cancer cells on their own but have some difficulty recognizing the substance produced by tumors that provides information on what cells to target.
Researchers at Penn State developed a new technology that promotes these interactions between immune and cancer cells. They published their results in the Angewandte Chemie International Edition.
“We explored a novel method to engineer natural killer cells,” said Yong Wang, professor of biomedical engineering. “This technique significantly improved the recognition and killing of target cancer cells.”
The team synthesized artificial structures that could bind to the body’s immune cells. Called polyvalent antibody mimics (PAMs), these polymer structures consist of a DNA scaffold with many branches. Each branch terminates in a molecule that can identify tumor markers on the tumor cell surface.
The PAMs were formed on white blood cells adapted for killing virus-infected cells and tumor cells. The researchers found that the PAM, compared to a similar structure that lacked the branched units, greatly improved how well the white blood cells could recognize cancer cells. Beyond allowing for easier targeting of the cancer cells, PAMs also made the immune cells more efficient killers—the PAM-enhanced immune cells were more than 2.5 times more effective than their natural counterparts.
The new method is cheaper than conventional genetic engineering techniques for preparing cell-based treatment, Wang said, and can be completed within a few hours—something that’s impossible for genetic engineering. This novel method can, however, be combined with the conventional method to minimize such roadblocks, according to Wang.
“The enhanced efficiency of PAM-engineered immune cells will reduce the doses of cells needed for immunotherapy, thereby further decreasing the manufacturing time and cost,” Wang said.
Source: Read Full Article
