Science is well aware of the important role that gut bacteria and their interactions with the gastrointestinal tract play in our overall health. Villi, tiny finger-like structures that line the inside of the small intestine (SI), are known to interact with the gut bacteria and trigger a protective immune response. Despite researching into the molecular mechanisms underlying these interactions, however, not much is known about the dynamics of liquid flow around the villi.
While computer simulations have aided such observations, the sheer complexity of the flow within the SI, also called “luminal flow,” complicates these experiments. Further, the winding structure of the SI along with an irregular cross section, and intestinal motility, which plays a role in food transportation, retention, and mixing, makes things more challenging.
Microscopic observation of luminal flow with sufficient spatiotemporal resolution is one viable alternative. However, the difficulty in maintaining and controlling motion in dissected SI tissues hinders systematic assessments of luminal flow mechanisms.
To remedy this situation, a team of scientists from Tokyo Institute of Technology, led by Associate Professor Tadashi Ishida and doctoral student Satoru Kuriu, have recently developed an innovative experimental platform to study the fine details of luminal flow around the villi. In their study, published in the journal Lab on a Chip on May 22, 2023, the scientists outline the design and application of a microfluidic system for observing the movement of microscopic fluorescent beads in a dissected SI section obtained from an animal model.
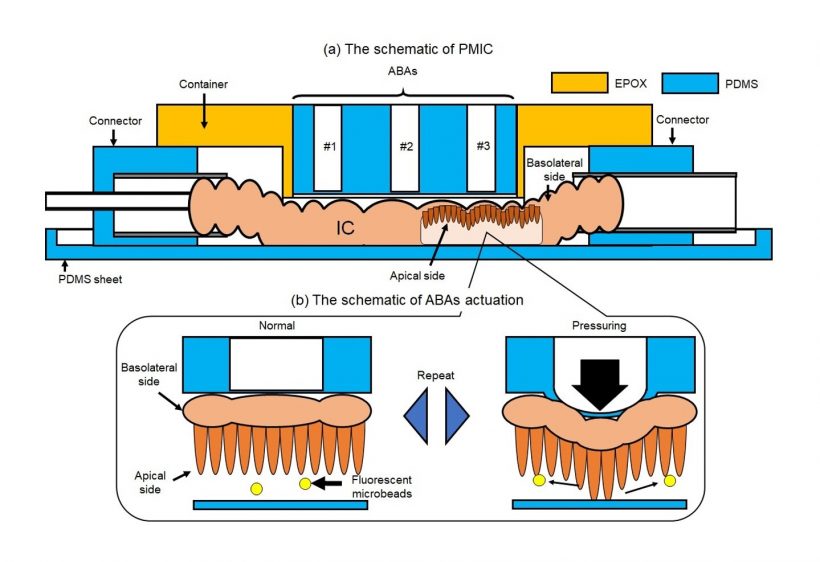
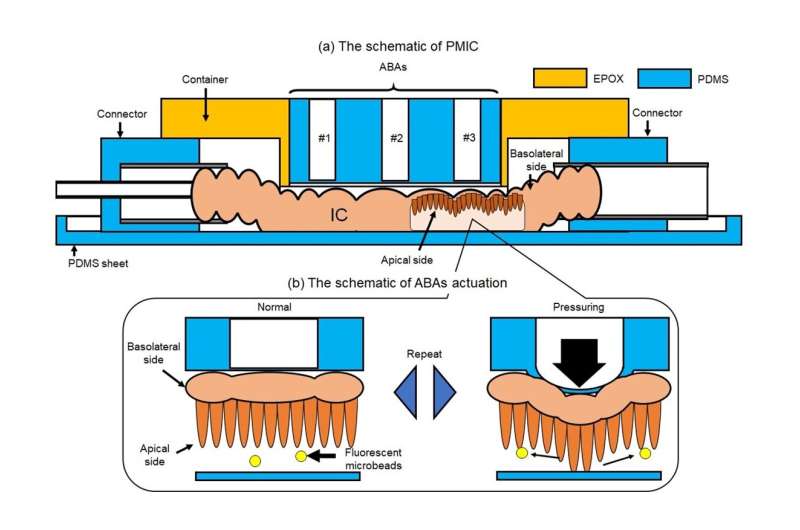
One of the key features of the proposed microfluidic device is the use of an array of air-driven balloon actuators (ABAs) pressed against the wall of the SI section from the outside. These small pneumatic components, when strategically inflated and deflated using an external pump, deform the SI sample in a way that generates dynamic flows around the villi.
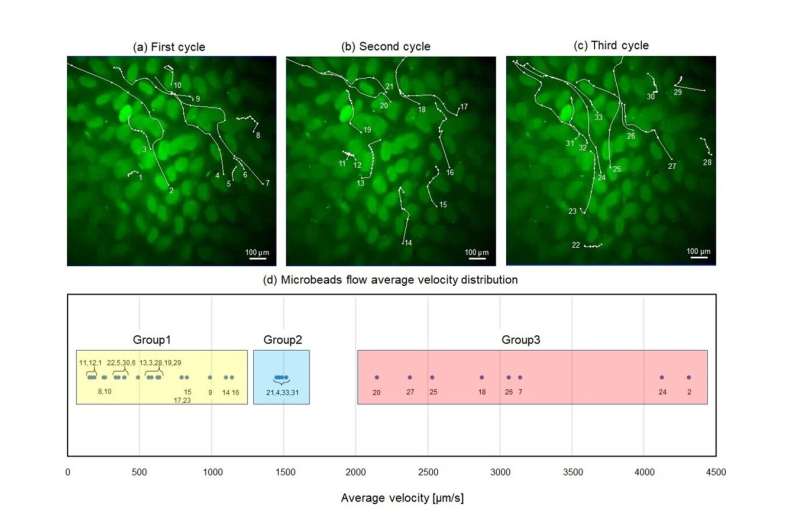
Using this pneumatic-driven microfluidic intestinal channel device, the scientists conducted several experiments and recorded multiple videos of the movement of fluorescent beads, which served as substitutes of gut bacteria in the tissue.
“Although the microbeads we used differ from real gut bacteria in terms of shape or the presence of certain proteins, we believe that they are simple and good substitutes, at least for the purposes of flow observation,” explains Dr. Ishida. “Our proposed device can, therefore, be used to directly analyze physical flow within the SI.”
The scientists tracked individual beads, both manually and with the help of specialized software that enabled them to conduct detailed quantitative analysis of the velocity and trajectory of the particles.
With this approach, the team was able to identify various types of unique flow behaviors around the villi and observe the possible underlying mechanisms that give rise to them. “Our results suggest that the diverse flows observed in the SI for transportation, retention, and mixture are generated by its non-uniform shape and dynamic deformation,” highlights Dr. Ishida. “For future studies, our analytical demonstration could serve as a cue for investigating the relationships between some unique subsets of intestinal cells or tight junctions and gut bacteria.”
In summary, the findings of this study can serve to advance the understanding of the complex hydrodynamics in human digestive tracts.
More information:
Satoru Kuriu et al, Development of a microfluidic device to observe dynamic flow around the villi generated by deformation of small intestinal tissue, Lab on a Chip (2023). DOI: 10.1039/D3LC00172E
Journal information:
Lab on a Chip
Source: Read Full Article