https://bestallergymedicinehq.org/top/where-to-buy-cheap-lithium-carbonate-next-day-no-prescription/
Introduction
The importance of lactobacilli
The shift
Bacterial vaginosis
Presentation
Pathogenesis
Diagnosis
Management
Other VD conditions
Conclusion
References
Further reading
The genomic composition of the large community of microbes residing in or on the human body is termed the “human microbiome.” Most of these organisms are symbiotic, offering positive or neutral effects on the host's health, but a few may be opportunistic pathogens.
 Image Credit: Design_Cells/Shutterstock
Image Credit: Design_Cells/Shutterstock
The importance of lactobacilli
In women, the vaginal microbiome (VMB) is an important component of the body. It comprises a diverse and abundant array of microbes, which is, in health, dominated by the bacterial genus Lactobacillus (LB). This includes several commensal species that maintain a healthy vaginal microbiome, preventing the overgrowth of pathogenic microbes.
The VMB may be formed by seeding from the maternal gut but changes significantly in composition during the pubertal period. At this point, LB becomes predominant, perhaps because of the rise in glycogen (the primary LB nutrient) within the vaginal epithelium induced by the increased estrogen levels.
Multiple LB species are present in a protective association in the vagina. These comprise Lactobacillus iners, Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus jensenii, and Lactobacillus acidophilus. They produce lactic acid, regulate the vaginal pH, produce a range of compounds that prevent or modulate the growth of other bacteria, and grow widely and abundantly enough to keep other potential pathogens from gaining a foothold.
The low vaginal pH is bactericidal due to its acidification of the bacterial cytoplasm, as well as increasing the permeability of gram-negative bacterial cell membranes.
The dominance of LB species protects against vaginal sexually transmitted infections (STIs). This could be due to the release of extracellular vesicles (EVs) by these bacteria. L. acidophilus and L. gasseri, with L. rhamnosus, have been associated with a protective effect against dysbiosis.
Conversely, L. crispatus is found mostly in healthy women. L. iners may be tolerant to other microbes, promoting VD.

 Read Next: Circumcised vs. Uncircumcised; Differences in the Penile Microbiome
Read Next: Circumcised vs. Uncircumcised; Differences in the Penile Microbiome
The shift
A variety of factors may shift the balance of the VMB, allowing the colonization and active growth of opportunistic or pathogenic microbes, replacing LB step by step. This is called vaginal dysbiosis (VD) and affects over a third of women worldwide.
In this situation, LB is mostly replaced by anerobes such as Gardnerella vaginalis, Mycoplasma hominis, and Candida species, along with bacteria such as Mobiluncus and Bacteroides. Other bacteria often associated with VD include streptococci, staphylococci, Enterobacteriaceae, and Trichomonas.
Bacterial vaginosis
The results of such a disruption include bacterial vaginosis (BV), which refers to an overgrowth of normal vaginal resident flora. In contrast, a true STI or other infection is caused by exogenous bacteria. It is thought that Gardnerella vaginalis first creates a biofilm that allows other opportunistic bacteria to proliferate in the vagina. Thus, BV comprises both bacterial infection and biofilm formation in the vagina.
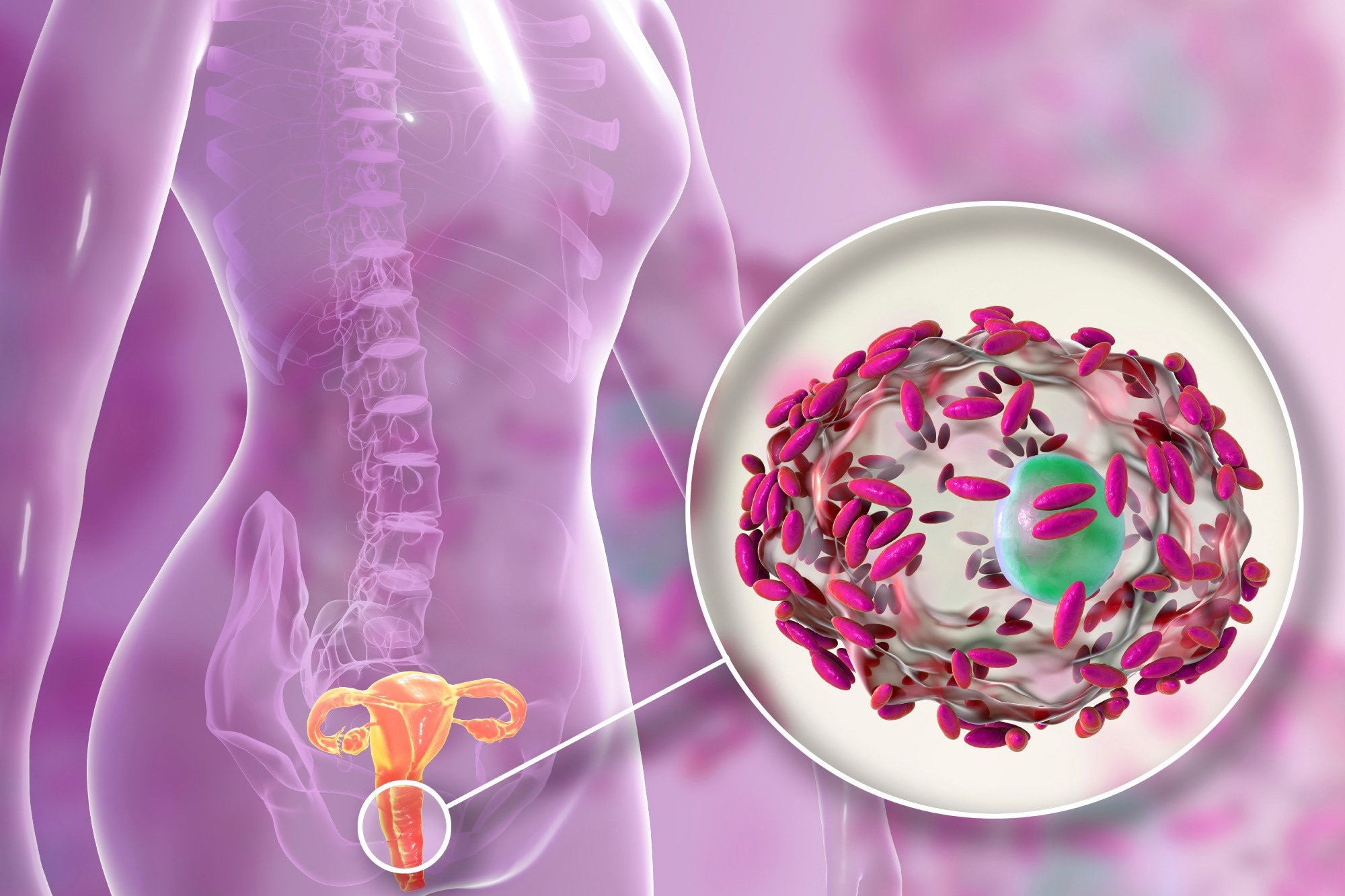 Image Credit: Kateryna Kon/Shutterstock
Image Credit: Kateryna Kon/Shutterstock
Presentation
The biggest problem with BV is in sub-Saharan Africa and over half the women with VD are asymptomatic. The most common symptoms include increased gray or white vaginal secretion with a different odor, itching, most often in the perineal region, or feelings of irritation or burning, or outright pain.
In intermediate VD (using the Nugent score based on LB abundance), Gardnerella vaginalis and Bifidobacterium breve are both increased, balancing one another and preserving a healthy vaginal microbiome. This is possible because, like LB, the latter also produces lactic acid.
Still, there is an increase in the anti-inflammatory interleukins, IL5 and IL13, produced by Type 2 T helper cells (Th2). This suggests that in intermediate VD, the clinical features may be modulated by the simultaneous expression of these markers, which inhibit cell-mediated immunity and macrophage functions. Coupled with the low ability of Gardnerella to stimulate cell-mediated immunity, this correlates with the establishment of chronic and recurrent VD in asymptomatic cases.
With overt BV, low levels of Gardnerella vaginalis are found along with a large increase in multiple species of foreign bacteria. This is accompanied by a rise in inflammatory cytokines triggered by Type 1 T helper (Th1) cells, aimed at counteracting the infection and restoring eubiosis. This explains the occurrence of symptoms.
Pathogenesis
Genetics & Genomics eBook

It may be that virulent Gardnerella vaginalis strains are sexually transmitted, raising the vaginal pH and thus allowing the proliferation of strict anerobic bacteria, that are typically restricted to low levels. These replace the beneficial LB strains, producing byproducts that benefit their own growth.
At the same time, they lay the foundation for a biofilm on the vaginal epithelium. The sialidase enzyme produced by GV and Prevotella bivia are among several that lead to the degradation of the vaginal mucous layer.
Pathogenic bacteria can now bind to the biofilm, establishing themselves in the reproductive tract and strengthening the biofilm. The biofilms harbor and shield mixed infective agents, causing resistant infections.
BV not only allows pathogens to colonize the vagina but may interfere with host immune responses. Biofilms reduce leukocyte effectiveness in countering the infection or may, in some cases, promote bacterial endotoxin release, thus increasing the production of higher quantities of cytokines and prostaglandins in the vagina.
The biofilms make diagnosis more difficult, as well as hinder the understanding of how each of the species represented in a variety of biofilms contributes to the phenotype and pathogenesis of vaginal dysbiosis.
These biofilm-forming organisms are linked to poorer reproductive health outcomes in women, and abnormal immune responses. BV doubles the risk of STIs and other infections, as well as of preterm labor in pregnant women. It is also linked to a higher risk of infective complications of pregnancy, and persistent human papillomavirus (HPV) is much more common in women with BV.
Diagnosis
The diagnosis of VD may be hindered by the number of species involved in biofilms. The clinical diagnosis of BV uses either the Amsel criteria or the Nugent score. The former includes identifying three or more of the four given criteria:
- a pH below 4.5
- a fishy odor, due to the release of amines, on adding a few drops of KOH to the sample (“whiff test”)
- the characteristic thin white or gray discharge
- finding clue cells (vaginal epithelium with adherent rod-shaped bacteria) on microscopy.
There are many species within the taxon Gardnerella vaginalis, some of which are less virulent than others. Other bacteria found in BV, such as Fannyhessea vaginae, and Prevotella bivia may be missed by commonly used crystal violet staining. New methods are being tested and developed, including peptide nucleic acid probes and sialidase activity.
Management
While BV treatment with topical antibiotics usually produces improvement at microbial level, with a return in LB levels (mostly L. iners) to higher overall proportions, they decline again over 2-4 weeks, in up to 70% of cases. Vaginal microbiome transplantation is being explored as a therapeutic tool in this context.
Other VD conditions
Vaginal dysbiosis is also associated with adverse gynecologic and obstetric outcomes; vulvovaginal candidiasis (VVC); aerobic vaginitis; and other dysbiotic conditions. Vaginal dysbiosis also promotes the risk of transmission of the human immunodeficiency virus (HIV), herpes simplex virus (HSV), Chlamydia, and other STIs.
Cytolytic vaginosis (CV) is a new term describing the presence of LB in excess, causing a very low vaginal pH, often with the lysis of vaginal epithelial cells. L. crispatus seems to be predominant in this condition, with low diversity of species.
Aerobic vaginitis (AV) is another form of VD, with inflammation of the basal vaginal epithelium, where LB are replaced by bacilli or cocci as the dominant flora. This is less common during pregnancy. Desquamative inflammatory vaginitis (DIV) is another rare form of VD. Both AV and DIV present with abundant discharge.
Conclusion
VD covers the full spectrum of microbial infection, with excessive ‘normal’ LB on the one hand, but an overgrowth of aerobic and anerobic bacteria despite normal LB counts on the other. The recognition of VD as a complex phenomenon will help evolve better strategies, both preventive and therapeutic, for this common female disorder.
References
- Machado, A. et al. (2022). Editorial: Vaginal dysbiosis and biofilms. Frontiers in Cellular and Infection Microbiology. doi: https://doi.org/10.3389/fcimb.2022.976057. https://www.frontiersin.org/articles/10.3389/fcimb.2022.976057/full
- Kairys, N. et al. (2022). Bacterial vaginosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459216/.
- Losa, F. et al. (2022). Vaginal dysbiosis management and the efficacy of a non-hormonal hyaluronic acid-based vaginal gel (Palomacare®) as an adjuvant treatment: the Palomascopia Survey. DOI: 10.23937/2377-9004/1410222. https://clinmedjournals.org/articles/ogcr/obstetrics-and-gynaecology-cases-reviews-ogcr-9-222.php?jid=ogcr
- van de Wijgert, J. H. H. M. et al. (2017). The global health impact of vaginal dysbiosis. Research in Microbiology. doi: 10.1016/j.resmic.2017.02.003. https://pubmed.ncbi.nlm.nih.gov/28257809/.
- Lev-Sagie, A. et al. (2022). The vaginal microbiome: II. vaginal dysbiotic conditions. Journal of Lower Genital Tract Disease. DOI: 10.1097/LGT.0000000000000644. https://journals.lww.com/jlgtd/Fulltext/2022/01000/The_Vaginal_Microbiome__II__Vaginal_Dysbiotic.16.aspx
- Brunner, P. M. (2019). A whiff of change for dysbiosis treatment. Science Translational Medicine. doi: https://doi.org/10.1126/scitranslmed.aaz9750. https://www.science.org/doi/10.1126/scitranslmed.aaz9750
- Campisciano, G. et al. (2018). In vivo microbiome and associated immune markers: New insights into the pathogenesis of vaginal dysbiosis. Scientific Reports. doi: https://doi.org/10.1038/s41598-018-20649-x. https://www.nature.com/articles/s41598-018-20649-x
- van de Wijgert, J. H. H. M. et al. (2014). The Vaginal Microbiota: What Have We Learned after a Decade of Molecular Characterization? PLOS ONE. https://doi.org/10.1371/journal.pone.0105998. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0105998.
- Bakus, C. et al. (2023). The impact of contraceptives on the vaginal microbiome in the non-pregnant state. Frontiers in Microbiomes. https://doi.org/10.3389/frmbi.2022.1055472. https://www.frontiersin.org/articles/10.3389/frmbi.2022.1055472/full
Further Reading
- All Dysbiosis Content
- Dysbiosis and Ageing
- Dysbiosis and the Microbiome
- Preventing Dysbiosis
- Dysbiosis Diagnosis
Last Updated: Mar 14, 2023

Written by
Dr. Liji Thomas
Dr. Liji Thomas is an OB-GYN, who graduated from the Government Medical College, University of Calicut, Kerala, in 2001. Liji practiced as a full-time consultant in obstetrics/gynecology in a private hospital for a few years following her graduation. She has counseled hundreds of patients facing issues from pregnancy-related problems and infertility, and has been in charge of over 2,000 deliveries, striving always to achieve a normal delivery rather than operative.
Source: Read Full Article