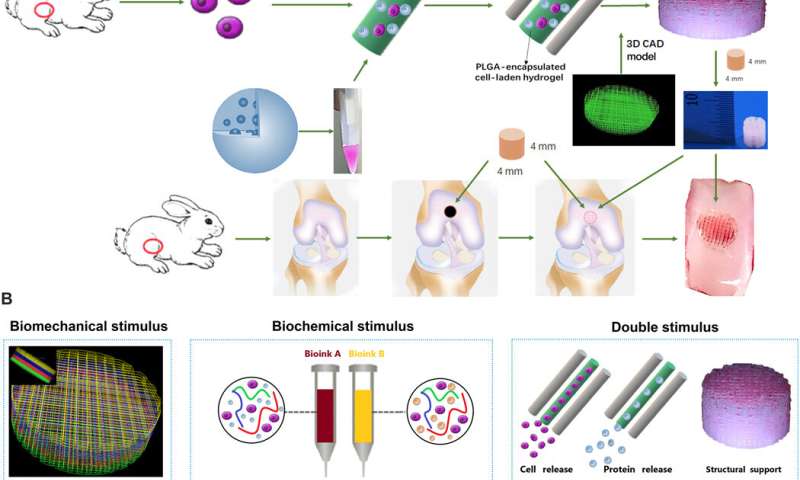
Cartilage injury is a common cause of joint dysfunction and existing joint prostheses cannot remodel with host joint tissue. However, it is challenging to develop large-scale biomimetic anisotropic constructs that structurally mimic native cartilage. In a new report on Science Advances, Ye Sun and a team of scientists in orthopedics, translational research and polymer science in China, detailed anisotropic cartilage regeneration using three-dimensional (3-D) bioprinting dual-factor releasing gradient-structured constructs. The team used the dual-growth-factor releasing mesenchymal stem cell (MSC)-laden hydrogels for chondrogenic differentiation (cartilage development). The 3-D bioprinted cartilage constructs showed whole-layer integrity, lubrication of superficial layers and nutrient supply into deeper layers. The scientists tested the cartilage tissue in the lab and in animal models to show tissue maturation and organization for translation to humans after sufficient experimental studies. The one-step, 3-D printed dual-factor releasing gradient-structured cartilage constructs can assist regeneration of MSC- and 3-D bioprinted therapy for injured or degenerative joints.
Chondrogenesis in the lab
Articular cartilage typically forms an elastic connective tissue in the joint. Cartilage injury is an extremely common impairment although with limited self-healing capacity due to the low cellularity and avascular nature of the tissue. Damage to cartilage can be debilitating and cartilage or joint reconstruction is currently considerably challenging in the research lab. In clinical practice arthritic joints can be replaced by total joint arthroplasty using metallic and synthetic prosthesis. However, the existing joint prostheses cannot remodel (or integrate) with host tissue, which can lead to long-term functional impairments that can only be addressed via biological regeneration of the joint. Scientists have recently developed mesenchymal stem cell (MSC) transplants to stimulate directional differentiation into chondrocytes as a new method for cartilage repair. However, it is still challenging to mimic the gradient anisotropic structure and signaling approaches in different layers to induce zonal-dependent chondrogenic differentiation and extracellular matrix (ECM) deposition to promote osteochondral regeneration. In this work, Sun et al. developed a 3-D bioprinted, dual-factor releasing, gradient-structured MSC-laden construct to implant and establish whole-layer cartilage regeneration in an animal model.
3-D bioprinting cartilage constructs
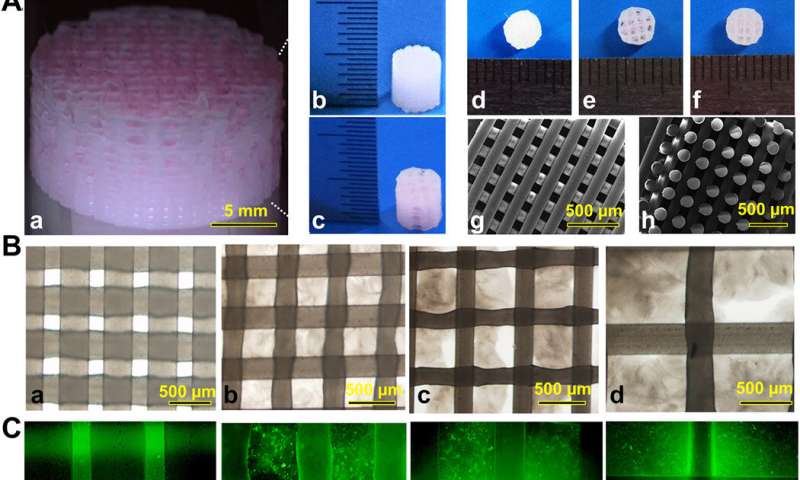
The team used 3-D bioprinting to develop different joint tissue constructs for joint reconstruction. They mimicked the native cartilage by including biochemical stimulus (BCS) with diverse growth factor releasing constructs and biomechanical stimulus (BMS) with small pore sizes to induce chondrogenesis. They then created a third cartilage construct as a double stimulus (DS) group to include the two versions of stimuli. For growth factors, the team chose a combination of bone morphogenetic protein (BMP4) and the transforming growth factor β3 (TGFβ3) in the cartilage construct to regenerate complex inhomogeneous joint tissues. Sun et al. then developed a hydrogel to deliver the growth factors and used poly(lactic-co-glycolic acid) (PLGA) microspheres as a carrier/vehicle. The team maintained constant fiber spacing for the BMS (biomechanical stimulus) group and BCS (biochemical stimulus) group to develop the non-gradient scaffolds, while introducing gradually varying fiber spacing for scaffolds in the DS (double stimulus) group. The scientists also used poly(ε-caprolactone) (PCL) polymers and integrated them to the biomimetic scaffold construct. In this way, they developed rabbit cartilage constructs using 4 x 4 x 4 mm scaffolds and human cartilage constructs using 14 x 14 x 14 mm scaffolds.

Testing the effects of the scaffolds and cartilaginous matrix formation in the lab
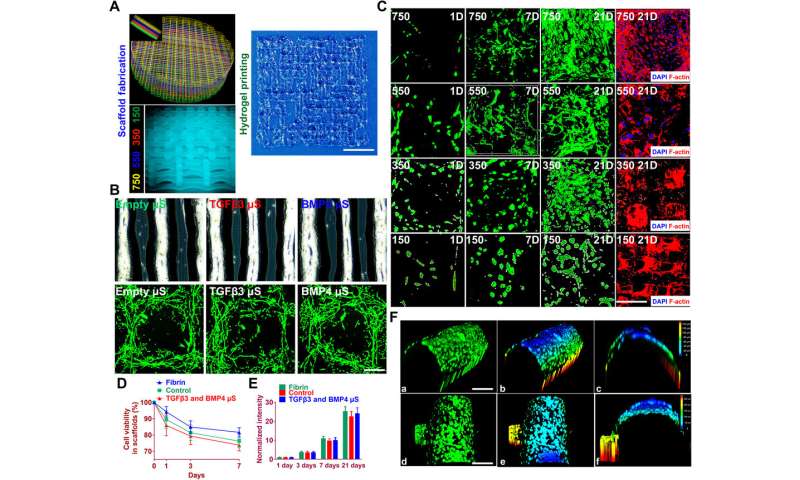
To test the impact of the growth factors on bone marrow stromal cell (BMSC) viability and proliferation, Sun et al. cultured BMSCs in hydrogels for seven days. The microspheres first released sustained and controlled volumes of growth factors in the lab. Then the scientists followed the experiment with cell viability and proliferation assays on printed scaffolds to note the survival of BMSCs at 60 minutes (day zero), seven days and 21 days after bioprinting. The live cells showed increased cell viability on day zero, followed by sustained growth from days three to 21. After 21 days, Sun et al. noted good 3-D cell anchorage on the scaffold and the work showed the development of a favorable microenvironment for BMSC growth and differentiation to form chondrocytes in the lab.
Before translating the 3-D bioprinted scaffold into an animal model, Sun et al. tested if the delivery of growth factors could induce layer-specific BMSC differentiation into chondrocytes. They followed an experimental protocol and observed aggrecan (a glial marker protein) and type II collagen to form hyaline articular chondrocyte-like cells. The team then transplanted the cartilage scaffolds into an animal model and examined their functionality for 12 weeks in vivo. The enhanced mechanical properties of the resulting articular cartilage showed promising regeneration to provide structural support for the newly formed cartilage tissue. After 12 weeks, Sun et al. conducted immunofluorescence imaging to show resemblance of the implanted cartilage constructs to native joint cartilage surrounded by the cartilage matrix.
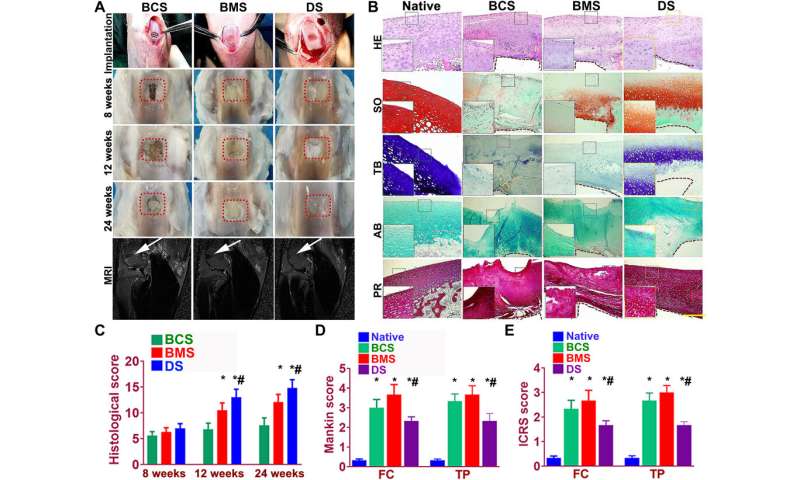
Better repairing effects of cartilage implants in a rabbit knee cartilage defect model
Sun et al. used rabbit experimental models to test the capacity of cartilage scaffolds during knee repair. They constructed the scaffolds using one-step 3-D bioprinting to provide structural support and sustained release of cells. The experiment facilitated biomimetic regeneration of the native articular cartilage and the implant showed better integration at the site of defect by 24 weeks for the dual stimuli (DS) experimental group compared with both BMS and BCS groups. The team monitored the site of transplantation with magnetic resonance imaging (MRI) for notable healing of the articular cartilage after 24 weeks (within six months). The results showed better cartilage repair and joint management with the DS cohort compared to the BCS or BMS animal groups.
Source: Read Full Article
