A “brain organoid” model using human cells matches molecular and pharmacological aspects of fragile X syndrome more closely than mouse versions, Emory scientists report. The results were published Thursday in Nature Neuroscience.
Fragile X syndrome is a leading genetic cause for intellectual disability and autism. Several drugs that appeared to work in mouse models of fragile X syndrome were not effective in human clinical trials, and researchers think that the “disease in a dish” model with human brain organoids could be a better way to find new potential treatments and evaluate them.
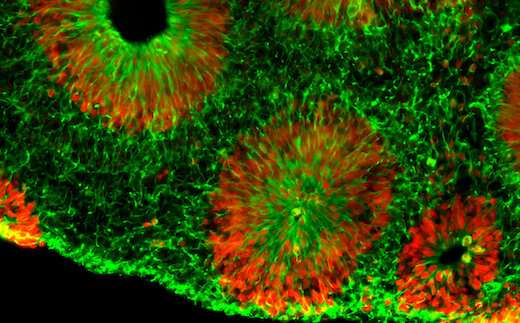
Using reprogrammed skin cells donated by patients with fragile X syndrome, scientists were able to mimic early stages of brain development in cell culture. The organoids correspond to a time 10 to 15 weeks after conception, when brain development is already perturbed by the fragile X mutation. The organoids contain cells from just one part of the brain—the forebrain—and are not considered to display consciousness.
“We think brain organoids could be a powerful way to test therapeutic approaches and develop new compounds,” says Peng Jin, Ph.D., chair of human genetics at Emory University School of Medicine and co-senior author of the paper. “We don’t have the opportunity to look at this stage of development directly in the human brain.”
Compared with control organoids, the fragile X organoids undergo accelerated differentiation and had fewer neurons that send inhibitory signals. In addition, the fragile X neurons were hyperexcitable, arranged into layers more quickly and tended to migrate in disorganized patterns.
Fragile X syndrome is caused by silencing of a single gene, preventing production of a critical regulatory protein, FMRP. Fragile X syndrome affects around 1 in 3000 males and 1 in 6000 females, with females often having milder symptoms. Affected children have intellectual disability, delay of milestones, and frequently autism-like features, such as impaired social skills, gaze aversion/social anxiety, and repetitive behaviors. No drugs that address the core molecular problems arising in fragile X syndrome are available for clinical use, although antipsychotic or antiseizure medications are sometimes prescribed.
In the Nature Neuroscience paper, the researchers say that the changes in development in the human brain organoids are “more pervasive” than in mouse models. They show that loss of the fragile X gene changed the activity of a larger number of genes in human brain cells, compared to mouse.
“These differences could be one of the reasons explaining why previous clinical trials were not successful,” says co-lead author Zhexing Wen, Ph.D., who brought the brain organoid model with him from Johns Hopkins when he joined Emory as a faculty member in 2016. The organoid model has also been used to study schizophrenia, Alzheimer’s disease and the effects of Zika virus infection on early brain development.
Postdoctoral fellows Yunhee Kang, Ph.D. and Ying Zhou, Ph.D. and instructor Yujing Li, Ph.D. are together the co-first authors of the paper. Kang and Li are in Jin’s lab, while Zhou is in Wen’s lab.
In the Nature Neuroscience paper, researchers tested a mGluR5 inhibitor, a drug that was tested in clinical trials based on promising results in mice. The mGluR5 inhibitor did not rescue developmental defects in the human organoid cultures.
In contrast, two other compounds that are PI3 kinase inhibitors—representing a strategy that is now being considered for clinical trials—did bring human brain organoids toward how controls look, in terms of the number of proliferating cells and the cells’ characteristics.
Source: Read Full Article
