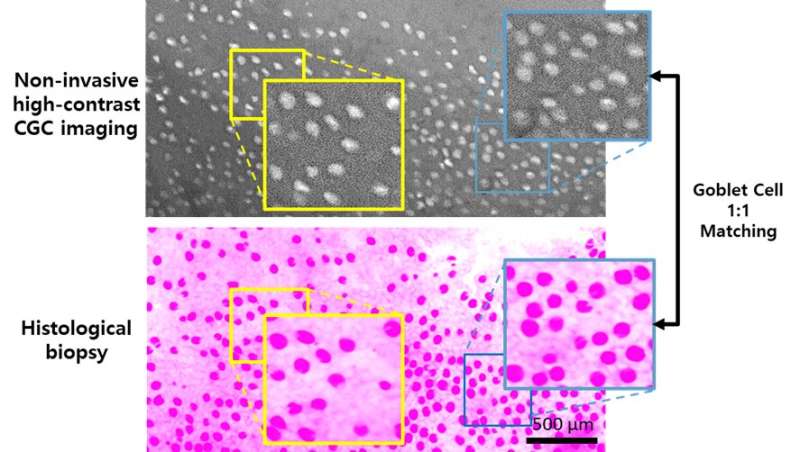
A Korean research team led by Professor Ki Hean Kim, Ph.D. candidates Seonghan Kim and Jungbin Lee (Department of Mechanical Engineering) at POSTECH, in collaboration with Professor Chang Ho Yoon (Department of Ophthalmology) of Seoul National University Hospital and Professor Young-Ho Jung (Department of Ophthalmology) of Eulji Hospital, has successfully demonstrated the non-invasive conjunctival goblet cell (CGC) examination in live rabbit eyes.
Goblet cells in the conjunctiva of eyes are specialized epithelial cells secreting mucins to form the mucus layer of tear film. The mucus layer spreads the tear film on the ocular surface for protection. The dysfunction and death of CGCs causes tear film instability and is associated with various ocular surface diseases, including dry eye disease (DED). Therefore, CGC examination is important for the precision diagnosis and effective treatment of ocular surface diseases. However, it has not been possible until now due to lack of non-invasive devices. The research team had recently developed non-invasive high-contrast CGC imaging methods, which use moxifloxacin, an FDA-approved ophthalmic antibiotic, as a cell labeling agent. In this study, the research team took one step forward by imaging CGCs in live rabbits whose eyes are similar to those of humans.
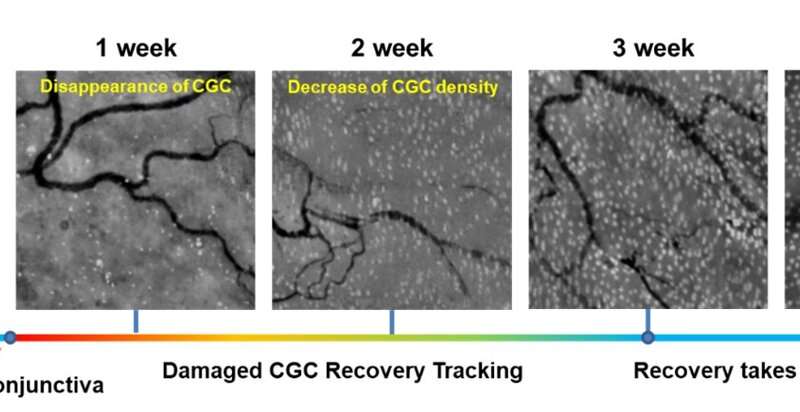
The researchers imaged normal rabbits first and verified that CGC images with the new imaging device were well matched with conventional histological images where CGCs were visible with periodic-acid Schiff (PAS) staining. The researchers then imaged DED rabbit models. DED was induced with topical instillation of povidone-iodine (product name: Betadine) which is a disinfectant used for ophthalmic surgery and is known for causing temporary DED after ophthalmic surgeries. The researchers observed the decrease of CGC density in the first and second weeks of DED induction, and then the recovery back to the normal level in the third and fourth weeks. The changes in CGC density observed with this technology in live rabbits were consistent with the results of standard DED evaluations including the corneal staining score, tear volume measurement (Schirmer’s test), and tear break-up time measurement, and the results of conventional histology with PAS staining.
The findings from the study were recently published in The Ocular Surface. Since this technology uses FDA-approved moxifloxacin antibiotic, it can be safely used in patients.
Source: Read Full Article
